This experimental cancer immunotherapy is designed for the treatment of non-small cell lung cancer (NSCLC).
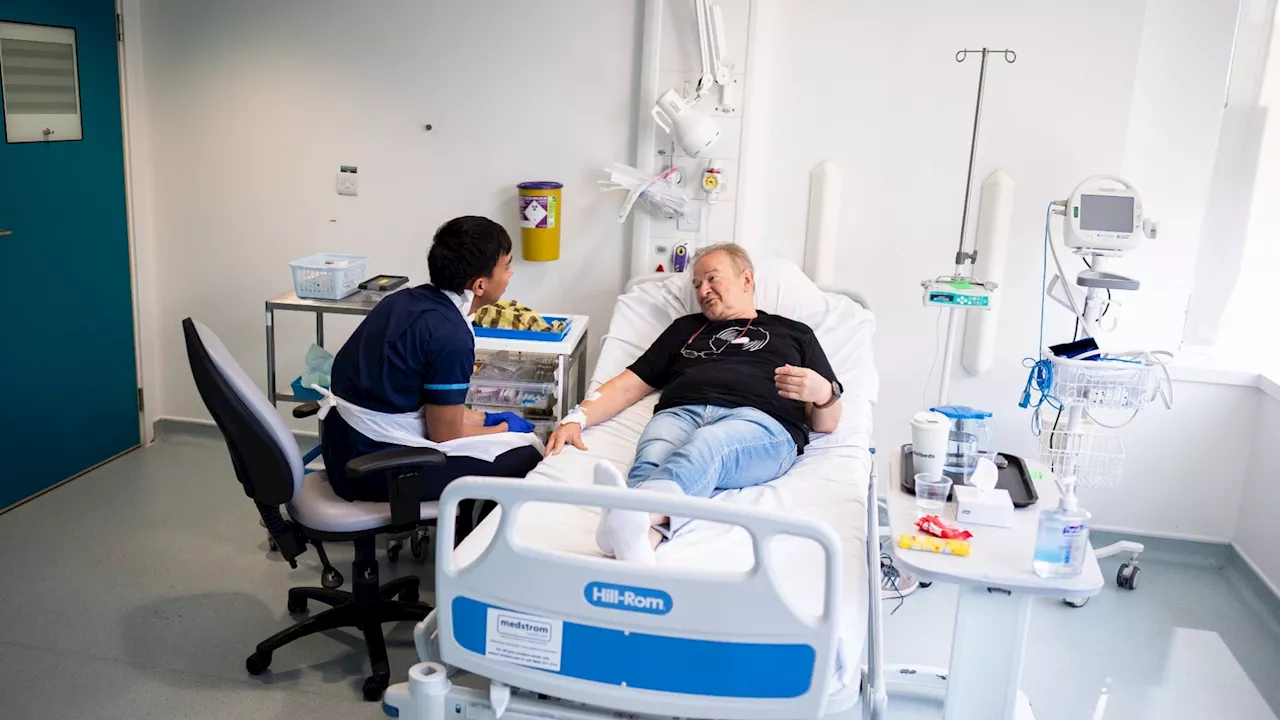
Janusz Racz, a 67-year-old lung cancer patient, is the first to receive this groundbreaking vaccine. He is part of a clinical trial that is taking place across multiple countries.
“We are now entering this very exciting new era of mRNA-based immunotherapy clinical trials to investigate the treatment of lung cancer,” said Siow Ming Lee, consultant medical oncologist from University College London Hospitals , who leads the national study. UCLH consultant medical oncologists Prof Siow-Ming Lee and Dr Sarah Benafif with trial participant Janusz Racz. Aaron Chown / PAIt uses messenger RNA to expose the patient’s immune system to NSCLC-associated tumor markers. This allows the immune system to identify and attack cancer cells that carry these markers.
This initial trial will establish the safety and tolerability of BNT116. The trial will enroll patients with NSCLC at various stages, from early-stage before surgery or radiotherapy, to late-stage disease or recurrent cancer.The study will enroll approximately 130 participants across 34 research sites in seven countries, six of which are in the United Kingdom. The other countries include the US, Germany, Hungary, Poland, Spain, and Turkey.
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA Calls AstraZeneca's NSCLC Trial Design Into QuestionThe agency says there's a problem with the latest trial of perioperative immunotherapy for NSCLC: There's no way to tell whether people need to continue treatment after surgery.
FDA Calls AstraZeneca's NSCLC Trial Design Into QuestionThe agency says there's a problem with the latest trial of perioperative immunotherapy for NSCLC: There's no way to tell whether people need to continue treatment after surgery.
Read more »
 IASLC Issues Consensus on Neoadjuvant, Adjuvant Treatments in Non–Small Cell Lung CancerMark Kris discusses adjuvant and neoadjuvant therapy consensus guidelines for NSCLC.
IASLC Issues Consensus on Neoadjuvant, Adjuvant Treatments in Non–Small Cell Lung CancerMark Kris discusses adjuvant and neoadjuvant therapy consensus guidelines for NSCLC.
Read more »
 Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?An obesity paradox does exist, but among patients with overweight or obesity, frontline immunotherapy was not associated with a survival benefit compared with chemotherapy, new data suggested.
Immunotherapy and Survival in Advanced NSCLC: Does Obesity Matter?An obesity paradox does exist, but among patients with overweight or obesity, frontline immunotherapy was not associated with a survival benefit compared with chemotherapy, new data suggested.
Read more »
 FDA Approves Neoadjuvant/Adjuvant Durvalumab for NSCLCThis approval follows recent concerns from an FDA committee that AstraZeneca did not demonstrate that patients needed the immunotherapy both before and after surgery.
FDA Approves Neoadjuvant/Adjuvant Durvalumab for NSCLCThis approval follows recent concerns from an FDA committee that AstraZeneca did not demonstrate that patients needed the immunotherapy both before and after surgery.
Read more »
 Does Pleural Invasion Lead to Worse Outcomes in Early NSCLC?Individuals with small, peripheral, node-negative NSCLC and visceral pleural invasion had higher recurrence rates than those without pleural involvement, but similar overall survival rates.
Does Pleural Invasion Lead to Worse Outcomes in Early NSCLC?Individuals with small, peripheral, node-negative NSCLC and visceral pleural invasion had higher recurrence rates than those without pleural involvement, but similar overall survival rates.
Read more »
 Chicago Cubs Legend and Hall of Famer Delivers Great Health Update in Battle w/ CanceThe news is looking all good for Chicago Cubs legend Ryne Sandberg!
Chicago Cubs Legend and Hall of Famer Delivers Great Health Update in Battle w/ CanceThe news is looking all good for Chicago Cubs legend Ryne Sandberg!
Read more »