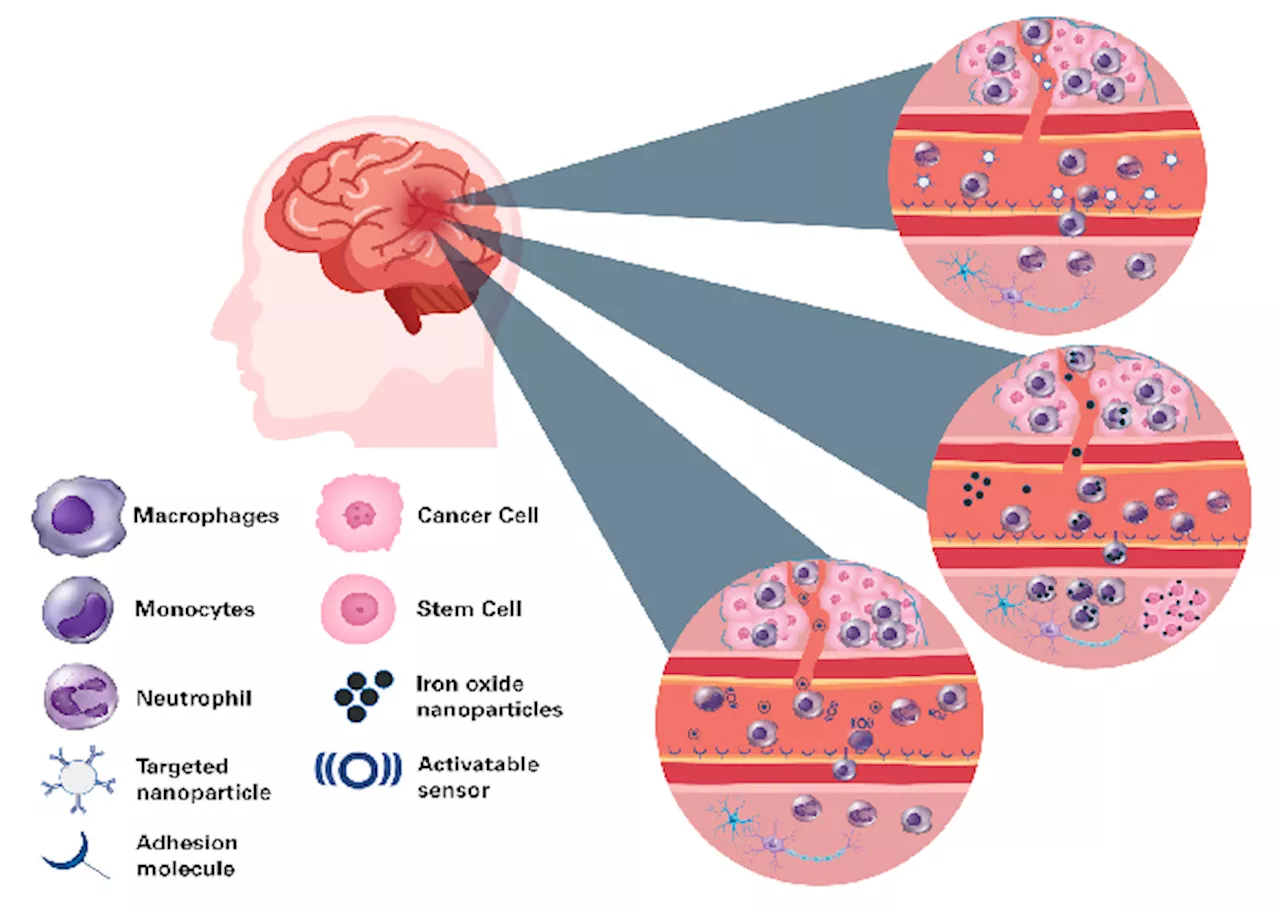
This article explores the crucial role of preclinical imaging tools in advancing research on neurological diseases. Bruker BioSpin's portfolio of MRI, PET, and SPECT imaging systems is highlighted for its ability to non-invasively visualize cellular and molecular processes in the central nervous system of animal models. The focus is on Bruker's MRI platform, including its BioSpec tools, and its applications in studying brain pathology, gene expression, and the efficacy of novel therapies.
Widespread neurological conditions, including Alzheimer's disease, Parkinson's disease, epilepsy, multiple sclerosis, stroke, and brain tumors, are among the top causes of disability and death around the world and bear a significant clinical, social, and economic burden.
Robust studies have unveiled complicated interactions among the molecular and cellular parts of nervous tissue, cerebral vasculature, and the immune system implicated in the genesis, progression, and maintenance of central nervous system (CNS) conditions. These processes' versatile character and interaction demand system approaches to research them inside an intact organism. Explorations are typically carried out in lab mice and rats due to both genetic and biological similarities to humans and the considerable variety of neurological disease conditions that can be triggered via experimental manipulation, genetic engineering, and changing lifestyle factors such as diet and environment. New therapeutic techniques, including immuno-, gene and cell therapy, brain-machine interfaces, and precise drug delivery approaches designed to reduce or cure neurological conditions, for which frequently few effective approaches exist, necessitate comprehensive preclinical evaluation in animal models before their translation to patients. Thus, approaches that non-invasively enable the visualization of cellular and molecular processes of the CNS in small animals are extremely sought-after. Bruker provides a portfolio of preclinical imaging tools, including magnetic resonance imaging (MRI), positron emission tomography (PET), and single photon emission computed tomography (SPECT), suitable for cellular and molecular imaging use cases. Among them, MRI has found significant application in examining CNS pathology, delivering data about changes in the anatomy, organization, and circuitry of the brain and spinal cord, and producing quantitative MRI measures of microstructure and tissue composition alongside metabolic and functional processes. Bruker’s dynamic MRI tool design includes BioSpec Maxwell tools beginning with 3 Tesla, classic MRIs, and ultra-high field MRIs like the BioSpec 18 Tesla. Tools are fitted with cutting-edge hardware parts from high-performance gradients to the electronic architecture to radiofrequency coils, committed to imaging the brain and spinal cord of both mice and rats alongside other model organisms, that collectively enable data collection both quickly and at a high quality, covering a wide variety of use cases. Bruker’s ParaVision software is accompanied by protocols that have been pre-optimized for small rodent species and associated use cases. It comprises sophisticated imaging viewing, reconstruction, and analysis options and a software framework for developing individual MR methods. Recent progress in disciplines, including chemistry, molecular and cell biology, and nanotechnology, has increased MRI’s capabilities to encompass the identification of pathological changes in tissue via instruments that visualize both cellular and molecular processes. Numerous novel imaging probe reporter genes and cell-labeling approaches form the foundations for gaining an unparalleled understanding of the pathomechanisms behind neurological conditions. In this paper, Bruker illustrates examples from academic work on the use of molecular and cellular MRI in models of neurological conditions alongside its use for evaluating both gene and cell therapies. Targeted MRI probes are developed to visualize specific pathophysiological molecular processes. They are typically composed of contrast agents like iron oxide nanoparticles, paramagnetic compounds (e.g., Gadolinium), chemical exchange saturation transfer (CEST)- and paramagnetic CEST (PARACEST)- agents, or perfluorocarbons (19F) and a targeting moiety like an antibody, peptide, oligonucleotide or enzyme substrate (Fig. 1). Microsized matrix-based magnetic particles (M3P) were utilized for detecting brain inflammation.1 M3Ps were fabricated via self-assembly of catechol-coated magnetite nanocrystals (Fig. 2A). The M3Ps have a high payload of superparamagnetic material and demonstrate biocompatibility and water dispersibility. M3Ps conjugated antibodies against the vascular cell adhesion molecule 1 (VCAM-1) to indicate endothelial activation. T2*-weighted MRI demonstrated local accumulation of VCAM-1-targeted M3P in the striatum following lipopolysaccharide injection, which led to focal neuroinflammation. M3P was additionally utilized to identify the inflammatory response to focal cerebral ischemia inside the mouse brain.
Magnetic Resonance Imaging (MRI) Preclinical Imaging Neurological Diseases Gene Therapy Cell Therapy Animal Models
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Envisioning Pathological Processes in the Central Nervous System Using Preclinical MRIThis article explores the use of magnetic resonance imaging (MRI) in preclinical neurological research. It highlights the role of MRI in understanding and studying various neurological conditions in animal models, emphasizing its contribution to drug development and validation of clinical imaging findings. The text focuses on Bruker's BioSpec Maxwell series as a powerful tool for preclinical MRI, featuring its different field strengths, coil options, and software capabilities. It also discusses advanced MRI techniques for metabolic and functional imaging, offering a deeper insight into pathological processes in the central nervous system.
Envisioning Pathological Processes in the Central Nervous System Using Preclinical MRIThis article explores the use of magnetic resonance imaging (MRI) in preclinical neurological research. It highlights the role of MRI in understanding and studying various neurological conditions in animal models, emphasizing its contribution to drug development and validation of clinical imaging findings. The text focuses on Bruker's BioSpec Maxwell series as a powerful tool for preclinical MRI, featuring its different field strengths, coil options, and software capabilities. It also discusses advanced MRI techniques for metabolic and functional imaging, offering a deeper insight into pathological processes in the central nervous system.
Read more »
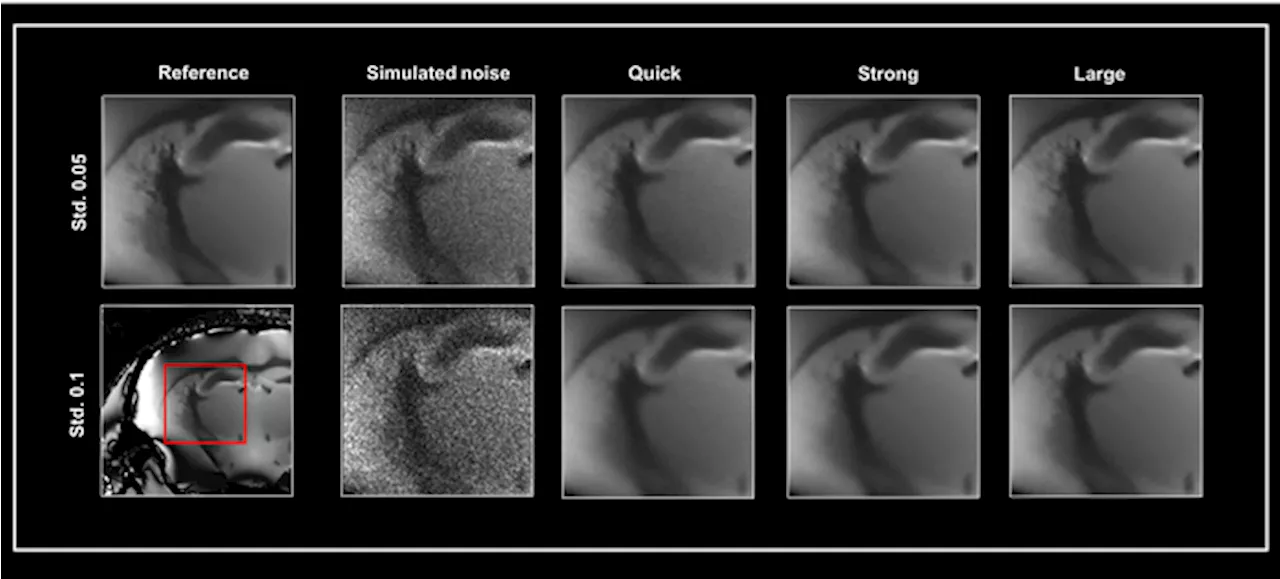 Smart Noise Reduction: Enhancing MRI Image Quality with AIBruker BioSpin's Smart Noise Reduction utilizes AI-powered algorithms to effectively remove noise from MRI images, improving image quality and precision in preclinical research.
Smart Noise Reduction: Enhancing MRI Image Quality with AIBruker BioSpin's Smart Noise Reduction utilizes AI-powered algorithms to effectively remove noise from MRI images, improving image quality and precision in preclinical research.
Read more »
 Case Center for Imaging Research: Bridging Preclinical and Clinical AdvancementsThe Case Center for Imaging Research (CCIR) at Case Western Reserve University and University Hospitals – Cleveland Medical Center fosters a collaborative environment for researchers across multiple disciplines to utilize advanced imaging techniques for medical and scientific breakthroughs.
Case Center for Imaging Research: Bridging Preclinical and Clinical AdvancementsThe Case Center for Imaging Research (CCIR) at Case Western Reserve University and University Hospitals – Cleveland Medical Center fosters a collaborative environment for researchers across multiple disciplines to utilize advanced imaging techniques for medical and scientific breakthroughs.
Read more »
 Estrogen's role in female binge drinking revealed by preclinical studyThe hormone estrogen regulates binge drinking in females, causing them to 'pregame' – consume large quantities of alcohol in the first 30 minutes after it's offered, according to a preclinical study led by scientists at Weill Cornell Medicine.
Estrogen's role in female binge drinking revealed by preclinical studyThe hormone estrogen regulates binge drinking in females, causing them to 'pregame' – consume large quantities of alcohol in the first 30 minutes after it's offered, according to a preclinical study led by scientists at Weill Cornell Medicine.
Read more »
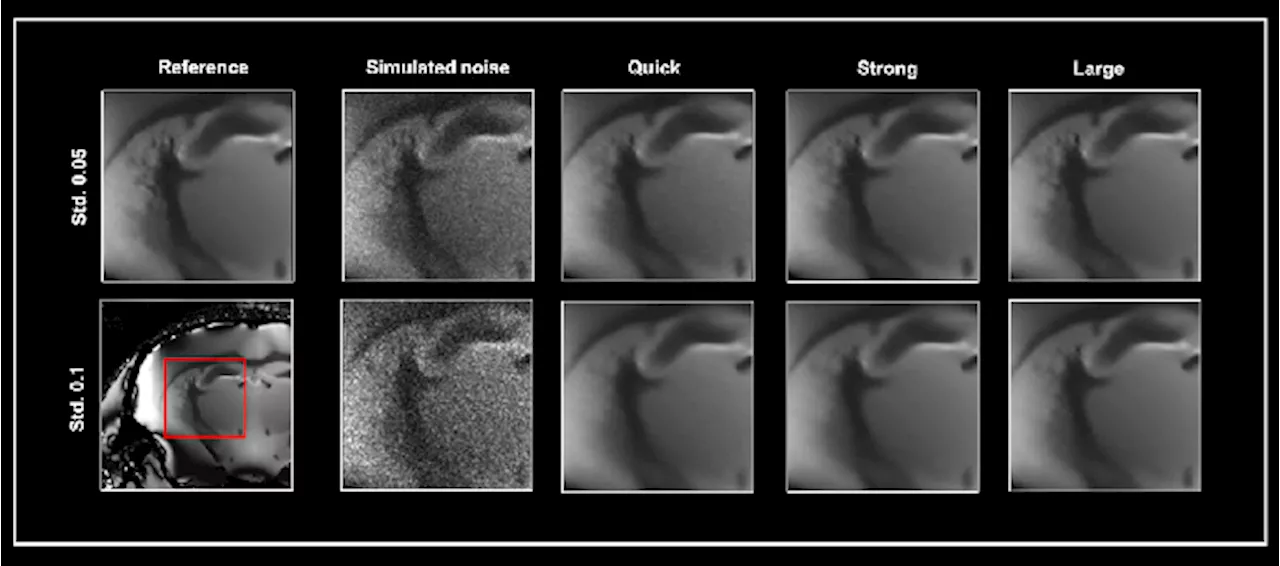 Improving noise reduction methods in structural MRI imagingDiscover an evaluation of the ParaVision 360 Smart Noise Reduction Algorithm and its impact on improving the quality of structural MR imaging.
Improving noise reduction methods in structural MRI imagingDiscover an evaluation of the ParaVision 360 Smart Noise Reduction Algorithm and its impact on improving the quality of structural MR imaging.
Read more »
 Advancing brain physiology and function measurements with UHF MRI techniquesDiscover how UHF MRI techniques are revolutionizing the measurement of brain physiology and function, offering insights into neural processes.
Advancing brain physiology and function measurements with UHF MRI techniquesDiscover how UHF MRI techniques are revolutionizing the measurement of brain physiology and function, offering insights into neural processes.
Read more »