Enterprise Therapeutics Ltd (Enterprise), a biopharmaceutical company dedicated to the discovery and development of novel therapies to improve the lives of people with respiratory diseases, today announced the publication of a peer reviewed study in the Journal of Cystic Fibrosis1.
Jun 12 2024Enterprise Therapeutics Ltd Enterprise Therapeutics Ltd , a biopharmaceutical company dedicated to the discovery and development of novel therapies to improve the lives of people with respiratory diseases, today announced the publication of a peer reviewed study in the Journal of Cystic Fibrosis 1. The paper describes low doses of the ENaC blocker ETD001, Enterprise’s lead asset, enhancing airway mucus clearance with an exceptionally long duration of action in a sheep model.
This study indicates that ETD001, at a dose level that was well tolerated in healthy volunteer studies, provides an opportunity to test whether a long-acting ENaC blocker can deliver benefit to people with cystic fibrosis . ETD001 is scheduled to commence a Phase 2 clinical study in pwCF in summer 2024, to understand whether 28 days of treatment will improve lung function.
Blocking ENaC in the airways offers a novel approach to improve mucus clearance in pwCF, including in the more than 10% of individuals who are either intolerant of or genetically unsuited to CFTR modulators. Recently, several other ENaC blocking drugs failed to show any benefit in clinical trials.
This data provides strong evidence that ETD001 has a superior profile compared to other inhaled ENaC blockers. Along with the results from the Phase 1 trials where ETD001 was well-tolerated, this study supports our high level of confidence going into the Phase 2 clinical trial. We remain passionate in our drive to discover novel therapies that have the potential to transform the lives of all people with cystic fibrosis.
Therapeutics Biopharmaceutical Cystic Fibrosis Fibrosis Respiratory
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
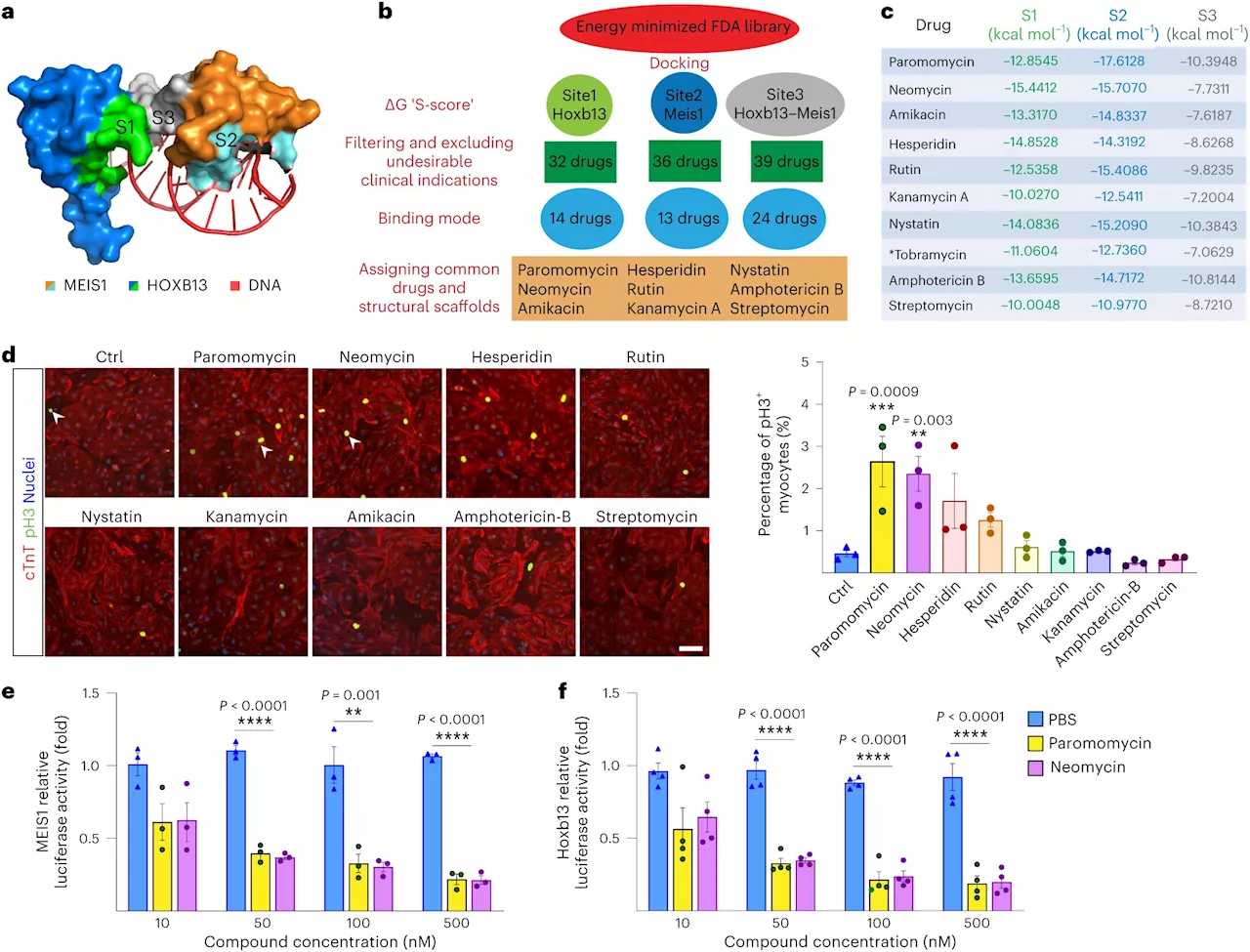 Study investigates cardiac cell regeneration in search of novel therapeuticsWhen a patient is experiencing heart failure, a leading cause of death worldwide, they begin to lose healthy and functioning cardiac cells. Heart failure causes these once-flexible cells to develop into fibrotic cells that are no longer able to contract and relax.
Study investigates cardiac cell regeneration in search of novel therapeuticsWhen a patient is experiencing heart failure, a leading cause of death worldwide, they begin to lose healthy and functioning cardiac cells. Heart failure causes these once-flexible cells to develop into fibrotic cells that are no longer able to contract and relax.
Read more »
 The Potential of Peptide Therapeutics in Treating Chronic DiseasesPeptide therapeutic research into chronic disease management led to the development of glucagon-like peptide-1 (GLP-1), which is produced by ileal endocrine cells and can stimulate insulin secretion.
The Potential of Peptide Therapeutics in Treating Chronic DiseasesPeptide therapeutic research into chronic disease management led to the development of glucagon-like peptide-1 (GLP-1), which is produced by ileal endocrine cells and can stimulate insulin secretion.
Read more »
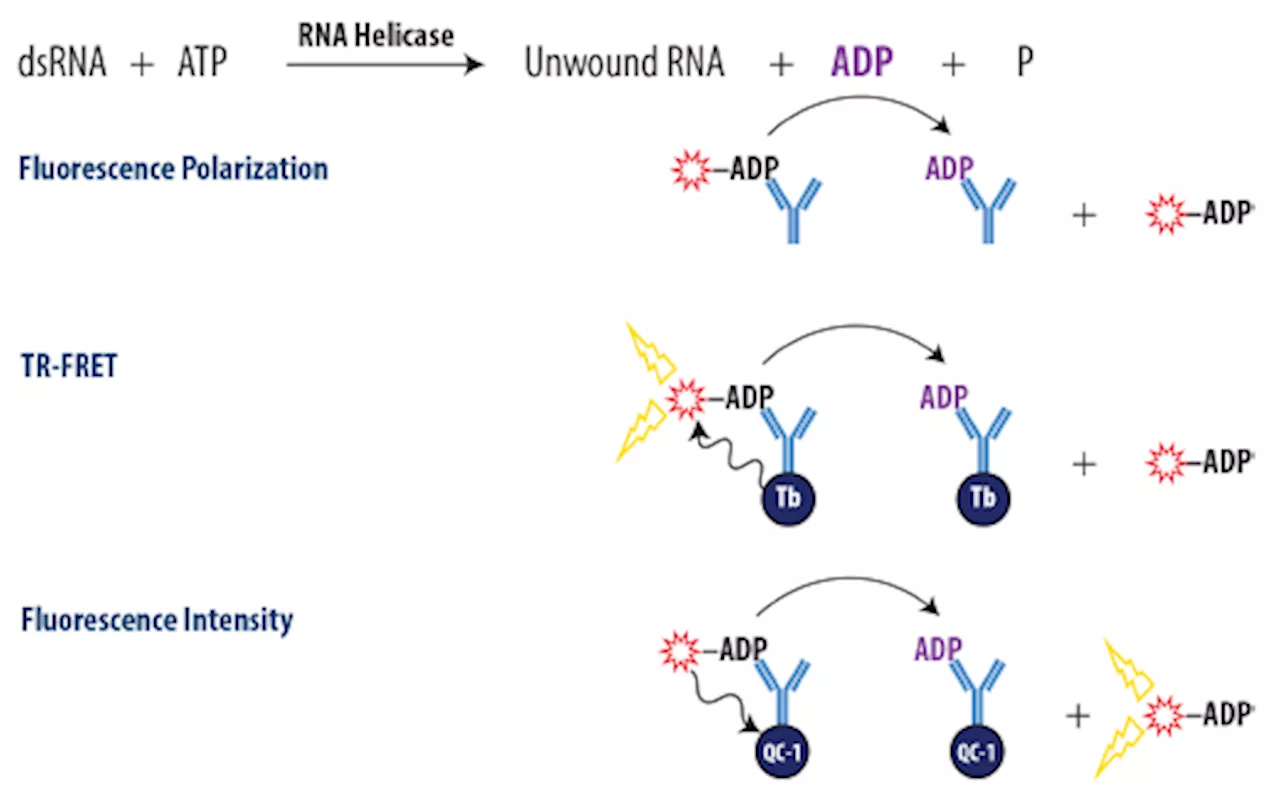 Advancing cancer therapeutics: Development of assays for measuring RNA helicase activityThis article explores advancing cancer therapeutics and the development of selective RNA helicase inhibitors.
Advancing cancer therapeutics: Development of assays for measuring RNA helicase activityThis article explores advancing cancer therapeutics and the development of selective RNA helicase inhibitors.
Read more »
 Sino Biological's cutting-edge recombinant cytokines for research and therapeuticsThis product profile highlights the cutting-edge recombinant cytokines offered by Sino Biological, engineered for advancing research and therapeutics in fields like tumor immunotherapy, stem cell therapy, and drug screening.
Sino Biological's cutting-edge recombinant cytokines for research and therapeuticsThis product profile highlights the cutting-edge recombinant cytokines offered by Sino Biological, engineered for advancing research and therapeutics in fields like tumor immunotherapy, stem cell therapy, and drug screening.
Read more »
 Three Lancashire firms among top UK businesses awarded with King's Award for EnterpriseThese three companies are being recognised for their brilliant achievements with a royal seal of approval!
Three Lancashire firms among top UK businesses awarded with King's Award for EnterpriseThese three companies are being recognised for their brilliant achievements with a royal seal of approval!
Read more »
 EMEA enterprise folks scrutinize deals more closely – and it's hurting WorkdayPesky 'macro' stuff forces SaaS biz to yank revenue forecast, share price plunges double digits
EMEA enterprise folks scrutinize deals more closely – and it's hurting WorkdayPesky 'macro' stuff forces SaaS biz to yank revenue forecast, share price plunges double digits
Read more »
