Eli Lilly's donanemab has received FDA approval as only the second therapy of its kind, backed unanimously by top experts, endorsing its use in early-stage Alzheimer's patients and highlighting the drug's favourable risk-benefit profile.
Eli Lilly's donanemab has received FDA approval as only the second therapy of its kind, backed unanimously by top experts, endorsing its use in early-stage Alzheimer's patients and highlighting the drug's favourable risk-benefit profile.
Like Eisai and Biogen's rival drug Leqembi, which was approved a year ago, donanemab is designed to clear an Alzheimer's-related protein called beta amyloid from the brain. BMO analyst Evan Seigerman said the price reflects the fact that patients can stop treatment versus chronic treatment with Leqembi.
Turkey Middle East Africa Europe China Us Germany News Breaking News Context Explainer Opinion Economy Business Trends Humans Globalisation Russia Rt Trt Trt World Journalism Human Interest
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 FDA analysis raises no major concerns about Eli Lilly Alzheimer's drugAn FDA analysis of trial data for Eli Lilly's experimental Alzheimer's drug donanemab released on Thursday revealed no red flags, but raised questions about safety of the treatment for patients with early-stage disease.
FDA analysis raises no major concerns about Eli Lilly Alzheimer's drugAn FDA analysis of trial data for Eli Lilly's experimental Alzheimer's drug donanemab released on Thursday revealed no red flags, but raised questions about safety of the treatment for patients with early-stage disease.
Read more »
 FDA advisors recommend Eli Lilly's Alzheimer's drug donanemab, paving way for approvalIf approved, Eli Lilly’s donanemab would become the second Alzheimer’s drug of its kind to enter the market after Leqembi from Biogen and Eisai.
FDA advisors recommend Eli Lilly's Alzheimer's drug donanemab, paving way for approvalIf approved, Eli Lilly’s donanemab would become the second Alzheimer’s drug of its kind to enter the market after Leqembi from Biogen and Eisai.
Read more »
 FDA advisors recommend Eli Lilly's Alzheimer's drug donanemab, paving way for approvalIf approved, Eli Lilly's donanemab would become the second Alzheimer's drug of its kind to enter the market after Leqembi from Biogen and Eisai.
FDA advisors recommend Eli Lilly's Alzheimer's drug donanemab, paving way for approvalIf approved, Eli Lilly's donanemab would become the second Alzheimer's drug of its kind to enter the market after Leqembi from Biogen and Eisai.
Read more »
 FDA advisors recommend Eli Lilly Alzheimer’s drug that slows diseaseEli Lilly's new Alzheimer's drug, donanemab, received approval from an advisory panel for the FDA. The full agency is expected to approve the drug by spring.
FDA advisors recommend Eli Lilly Alzheimer’s drug that slows diseaseEli Lilly's new Alzheimer's drug, donanemab, received approval from an advisory panel for the FDA. The full agency is expected to approve the drug by spring.
Read more »
 Alzheimer’s drug from Eli Lilly wins backing of FDA committeeAn advisory committee to the Food and Drug Administration found the drug was safe and effective at slowing the progression of the disease, setting the stage for the agency to approve it.
Alzheimer’s drug from Eli Lilly wins backing of FDA committeeAn advisory committee to the Food and Drug Administration found the drug was safe and effective at slowing the progression of the disease, setting the stage for the agency to approve it.
Read more »
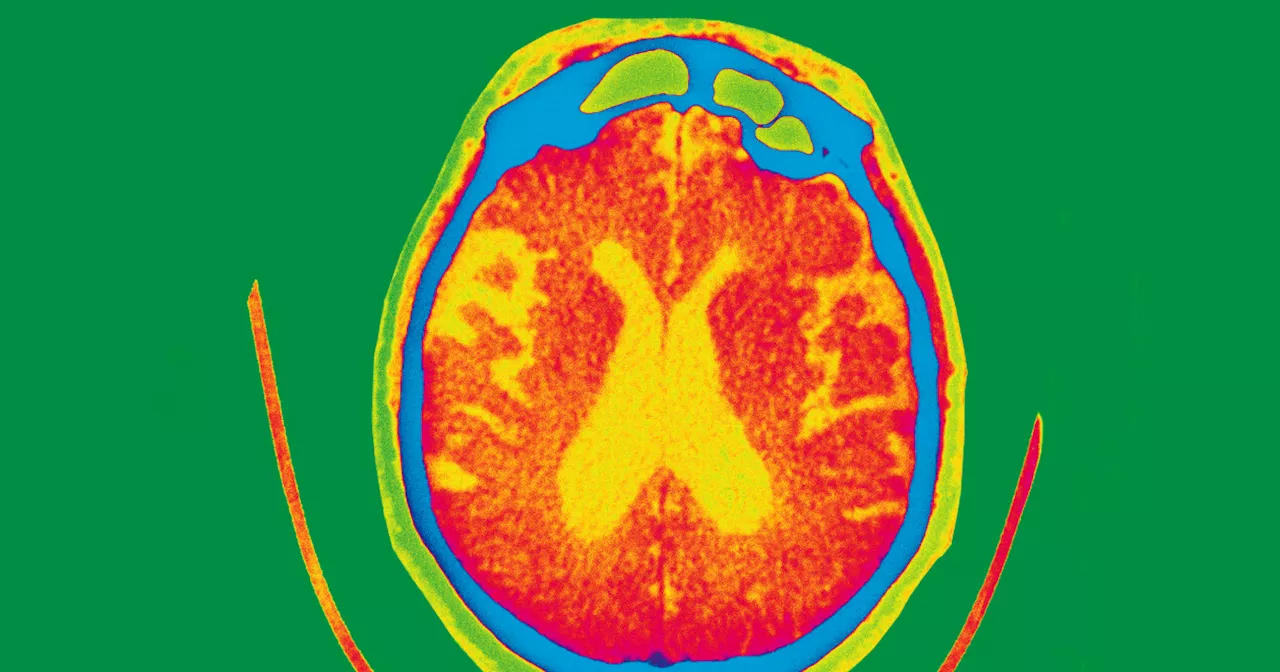 FDA approves Eli Lilly's Alzheimer’s drug that slows memory declineBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
FDA approves Eli Lilly's Alzheimer’s drug that slows memory declineBerkeley Lovelace Jr. is a health and medical reporter for NBC News. He covers the Food and Drug Administration, with a special focus on Covid vaccines, prescription drug pricing and health care. He previously covered the biotech and pharmaceutical industry with CNBC.
Read more »
