Cell therapies have been getting a great deal of attention as of late—most notably, gene-modified cell therapies for cancer immunology, such as CAR T therapies.
Sponsored Content by Corning Life SciencesNov 22 2022Reviewed by Olivia Frost Diseases for which there are frequently no effective treatments can potentially be treated and sometimes cured with cell therapies. Several different therapeutic modalities are covered by the term “cell therapy”, employing various cell types and manufacturing processes, and targeting a variety of conditions.
With 1,220 ongoing clinical trials worldwide, cell and gene therapies are moving forward in the clinical pipeline at the fastest rate so far.1 The need for manufacturing solutions has grown due to this market expansion, strong clinical outcomes, and recent regulatory approvals.Indications In particular, gene-modified cell therapies for cancer immunology, such as CAR T therapies, have received much attention recently.
The first treatment authorized by the FDA under the Regenerative Medicine Advanced Therapy Designation was Breyanzi. Regenerative medicine therapies intended for serious conditions can be developed more quickly with the help of the RMAT designation. On the other hand, allogeneic therapies use tissue from unrelated donors and are produced in large batches. As a single product used to treat many patients, allogeneic therapies are regarded as being “off the shelf.” Scaling up the manufacturing vessel volume is necessary for these therapies.
The general cellular therapy workflow includes upstream and downstream unit operations such as cell isolation, cell culture media optimization, cell expansion, modification, purification, and characterization. However, the specific process steps can vary.Media optimization Since the therapeutic product in cell-based therapies is a cultured cell, cell culture media must be optimized to meet growth and productivity goals for cell production.
Knowing how much material is required to produce the therapeutic means deciding whether to scale up or out. No matter the manufacturing process, the cells must maintain their phenotype and functionality since they are the therapeutic product.
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 CAR T-cell therapy in colorectal cancerCAR T-cell therapy in colorectal cancer FrontiersIn FrontImmunol CARTCell therapy immunology immunotherapy TCell Cancer ColorectalCancer
CAR T-cell therapy in colorectal cancerCAR T-cell therapy in colorectal cancer FrontiersIn FrontImmunol CARTCell therapy immunology immunotherapy TCell Cancer ColorectalCancer
Read more »
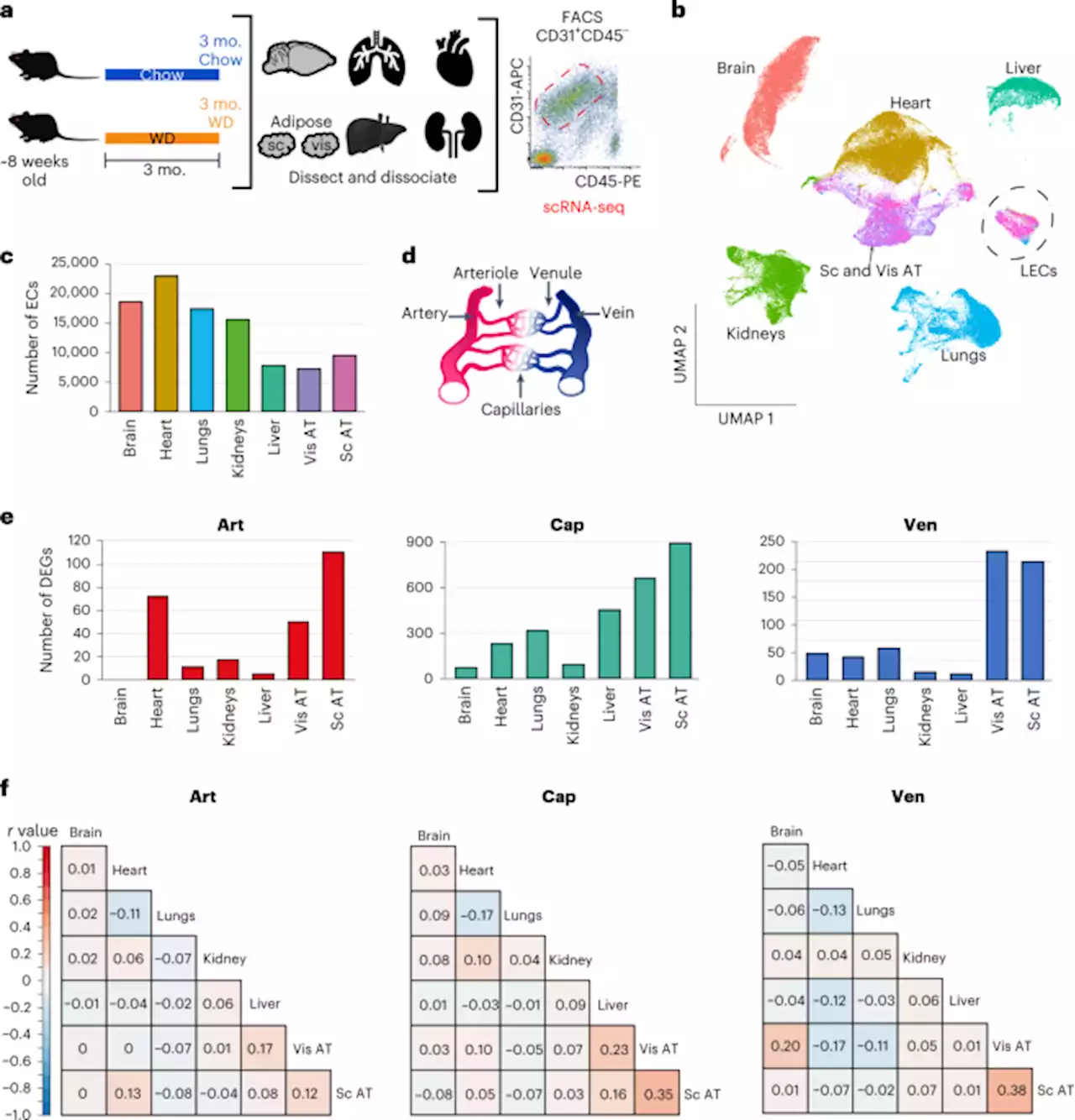 Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity - Nature MetabolismBondareva and Rodríguez-Aguilera et al. use scRNA-seq to analyze transcriptomes of 375,000 endothelial cells from seven organs in male mice at various stages of obesity to identify organ-specific vulnerabilities.
Single-cell profiling of vascular endothelial cells reveals progressive organ-specific vulnerabilities during obesity - Nature MetabolismBondareva and Rodríguez-Aguilera et al. use scRNA-seq to analyze transcriptomes of 375,000 endothelial cells from seven organs in male mice at various stages of obesity to identify organ-specific vulnerabilities.
Read more »
 Intestinal microorganisms influence white blood cell levels, study findsIntestinal bacteria composition is crucial to driving the recovery of neutrophil counts in the blood of mice following treatments such as stem cell transplants or chemotherapy.
Intestinal microorganisms influence white blood cell levels, study findsIntestinal bacteria composition is crucial to driving the recovery of neutrophil counts in the blood of mice following treatments such as stem cell transplants or chemotherapy.
Read more »
 Evaluating cell-free DNA-based blood tests for early detection of multiple cancersResearchers assessed several approaches for a circulating cell-free deoxyribonucleic acid (cfDNA)-based multi-cancer early detection (MCED) test. Defining the clinical limit of detection (LOD) based on circulating tumor allele fraction (cTAF) enables the comparison of different approaches.
Evaluating cell-free DNA-based blood tests for early detection of multiple cancersResearchers assessed several approaches for a circulating cell-free deoxyribonucleic acid (cfDNA)-based multi-cancer early detection (MCED) test. Defining the clinical limit of detection (LOD) based on circulating tumor allele fraction (cTAF) enables the comparison of different approaches.
Read more »
 Ubisoft shares Splinter Cell remake update and new concept artSplinter Cell has just had its 20th anniversary and to celebrate, Ubisoft has shared a video discussing the series so far, as well as revealing some concept art and details about the Splinter Cell remake.
Ubisoft shares Splinter Cell remake update and new concept artSplinter Cell has just had its 20th anniversary and to celebrate, Ubisoft has shared a video discussing the series so far, as well as revealing some concept art and details about the Splinter Cell remake.
Read more »
 All 'Warm Spaces' across West Northants to go and keep warm67 community groups, charities, and local societies across the area have opened their doors
All 'Warm Spaces' across West Northants to go and keep warm67 community groups, charities, and local societies across the area have opened their doors
Read more »