CDK4/6 inhibitors, which are already FDA approved for the treatment of other forms of cancer, show early signs of promise in the treatment of a subtype of pediatric high-grade glioma, according to new research from Dana-Farber Cancer Institute and the Institute of Cancer Research in London.
Dana-Farber Cancer InstituteSep 3 2024 Treatment of a patient with a second relapse of this glioma subtype and no other treatment options resulted in 18 months of progression-free survival.
Filbin and her team discovered, surprisingly, that the tumor cells more closely resemble neurons. The team made this discovery using single cell multi-omic sequencing – the analysis of the active genes and proteins in individual cells in the tumor samples. The screen's results also pointed to CDK6 as a key vulnerability. CDK6 is a gene that regulates the cell division cycle and is important in cell fate decisions as cells differentiate. Several CDK4/6 inhibitors are already approved for the treatment of other cancers such as breast cancer.
The team first confirmed that three CDK4/6 inhibitors, ribociclib, palbociclib, and abemaciclib, could penetrate the blood brain barrier. Ribociclib, however, had several advantages, including being better tolerated at higher concentrations and more specificity to CDK6. In mouse models with patient-derived xenografts, treatment with ribociclib slowed tumor growth and extended survival.
Adolescents Blood Brain Brain Cancer Brain Tumor Cancer Cell Children CRISPR Drugs Genes Gliomas Hospital Leukemia Neuron Neurons Neuro-Oncology Oncology Research Tumor
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
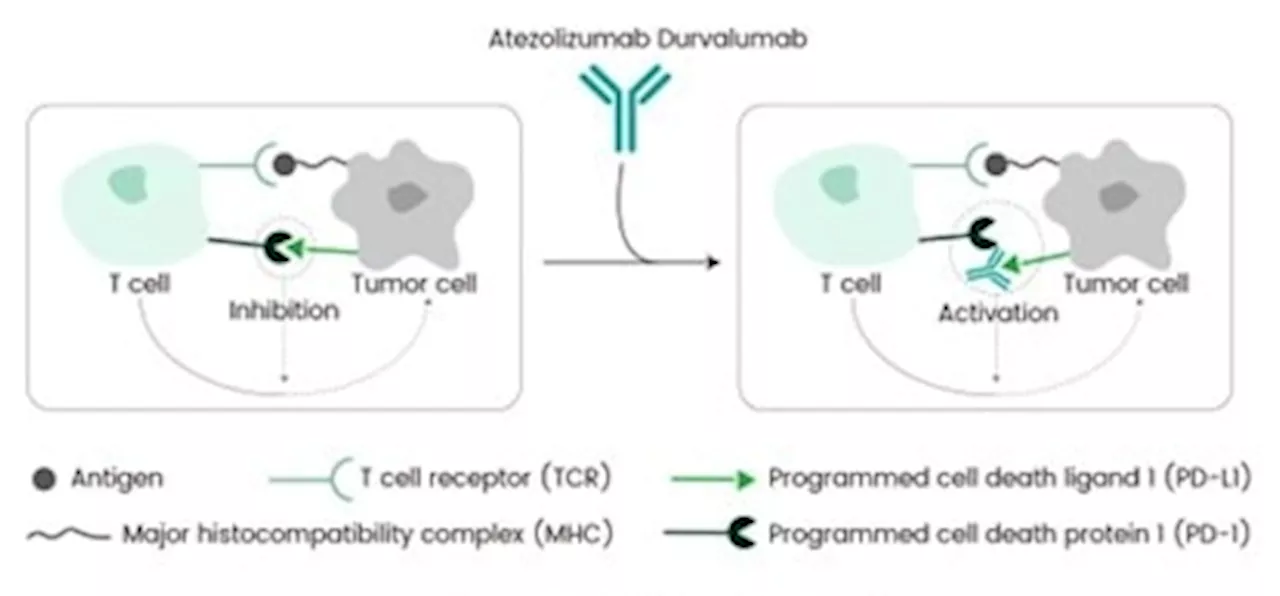 FDA accepted PD-L1 inhibitor durvalumab for the treatment of endometrial cancerOn June 14, 2024, the U.S. FDA approved AstraZeneca's Durvalumab (IMFINZI®) for primary advanced or recurrent endometrial cancer with mismatch repair deficiency. Endometrial cancer is the most prevalent gynecologic cancer, with an estimated 66,200 new U.S. cases in 2023.
FDA accepted PD-L1 inhibitor durvalumab for the treatment of endometrial cancerOn June 14, 2024, the U.S. FDA approved AstraZeneca's Durvalumab (IMFINZI®) for primary advanced or recurrent endometrial cancer with mismatch repair deficiency. Endometrial cancer is the most prevalent gynecologic cancer, with an estimated 66,200 new U.S. cases in 2023.
Read more »
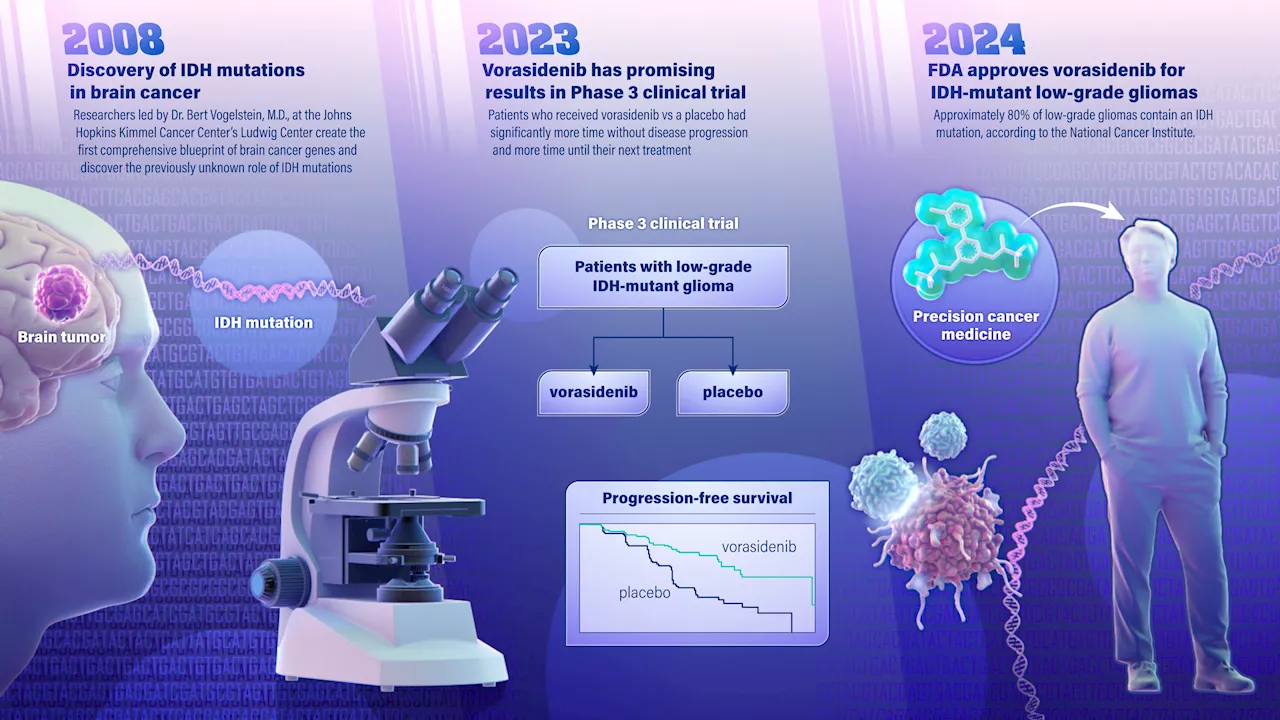 FDA approves drug targeting brain cancer gene mutationA new drug for treatment of a type of brain cancer, called IDH-mutant low-grade glioma, was approved Aug. 6 by the U.S. Food and Drug Administration (FDA). The promising new drug stems from a 2008 genetic discovery made at the Johns Hopkins Kimmel Cancer Center.
FDA approves drug targeting brain cancer gene mutationA new drug for treatment of a type of brain cancer, called IDH-mutant low-grade glioma, was approved Aug. 6 by the U.S. Food and Drug Administration (FDA). The promising new drug stems from a 2008 genetic discovery made at the Johns Hopkins Kimmel Cancer Center.
Read more »
 Joy as £127k raised from Dave Day in memory of Hairy Bikers' star Dave MyersThe money raised is split between charities the NSPCC and the Institute of Cancer Research.
Joy as £127k raised from Dave Day in memory of Hairy Bikers' star Dave MyersThe money raised is split between charities the NSPCC and the Institute of Cancer Research.
Read more »
 Study finds breast cancer screening attendance helps boost other cancer screeningsOffering self-sampling kits to women overdue for cervical cancer (CC) or colorectal cancer (CRC) screening when they attend breast cancer (BC) screening can result in increased screening participation, according to a study published online Aug. 13 in PLOS Medicine.
Study finds breast cancer screening attendance helps boost other cancer screeningsOffering self-sampling kits to women overdue for cervical cancer (CC) or colorectal cancer (CRC) screening when they attend breast cancer (BC) screening can result in increased screening participation, according to a study published online Aug. 13 in PLOS Medicine.
Read more »
 Targeted cancer cell therapy may slow endometrial cancerThere may be a way to slow the growth of endometrial cancer through targeted cancer cell therapy, according to new research from the University of Missouri School of Medicine.
Targeted cancer cell therapy may slow endometrial cancerThere may be a way to slow the growth of endometrial cancer through targeted cancer cell therapy, according to new research from the University of Missouri School of Medicine.
Read more »
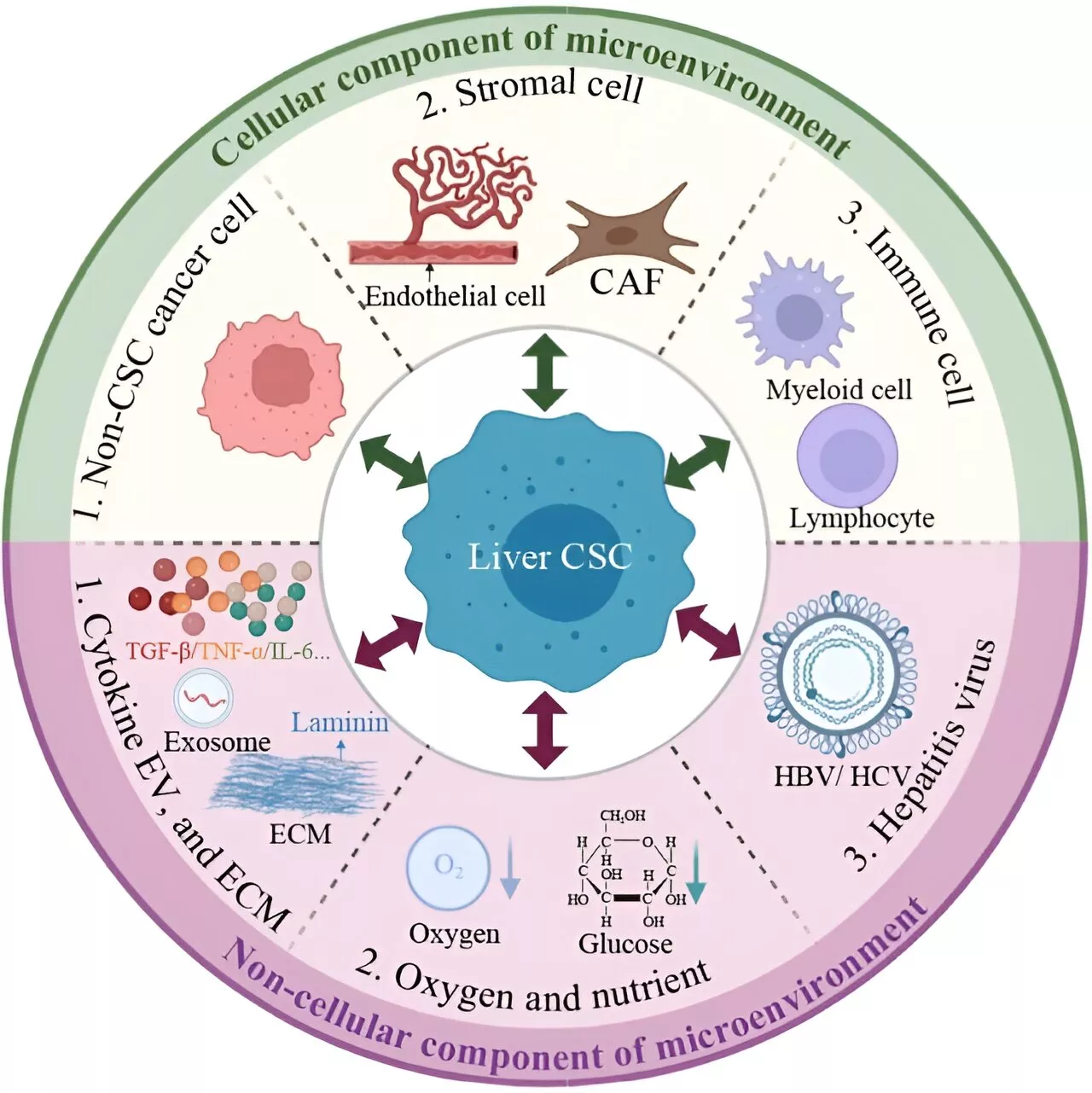 Cancer stem cell-immune cell crosstalk in the tumor microenvironment for liver cancer progressionThe complex dynamics between liver cancer stem cells (CSCs) and immune cells within the tumor microenvironment (TME) are central to the progression of liver cancer. These interactions are critical in creating an immunosuppressive setting that significantly impacts the response to immunotherapy.
Cancer stem cell-immune cell crosstalk in the tumor microenvironment for liver cancer progressionThe complex dynamics between liver cancer stem cells (CSCs) and immune cells within the tumor microenvironment (TME) are central to the progression of liver cancer. These interactions are critical in creating an immunosuppressive setting that significantly impacts the response to immunotherapy.
Read more »
