The U.S. Food and Drug Administration has approved the first generic version of Emflaza (deflazacort) oral suspension for Duchenne muscular dystrophy (DMD). Approval of the generic version of Emflaza oral suspension was granted to Cranbury Pharmaceuticals (Tris Pharma).
FDA approves generic Emflaza oral suspension for Duchenne muscular dystrophy retrieved 17 June 2024 from https://medicalxpress.com/news/2024-06-fda-generic-emflaza-oral-suspension.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no part may be reproduced without the written permission. The content is provided for information purposes only.Jun 14, 2024 Use this form if you have come across a typo, inaccuracy or would like to send an edit request for the content on this page. For general inquiries, please use ourThank you for taking time to provide your feedback to the editors.
Your feedback is important to us. However, we do not guarantee individual replies due to the high volume of messages.to let the recipient know who sent the email. Neither your address nor the recipient's address will be used for any other purpose. The information you enter will appear in your e-mail message and is not retained by Medical Xpress in any form.Get weekly and/or daily updates delivered to your inbox.
Medicine Research Health Research News Health Research Health Science Medicine Science
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
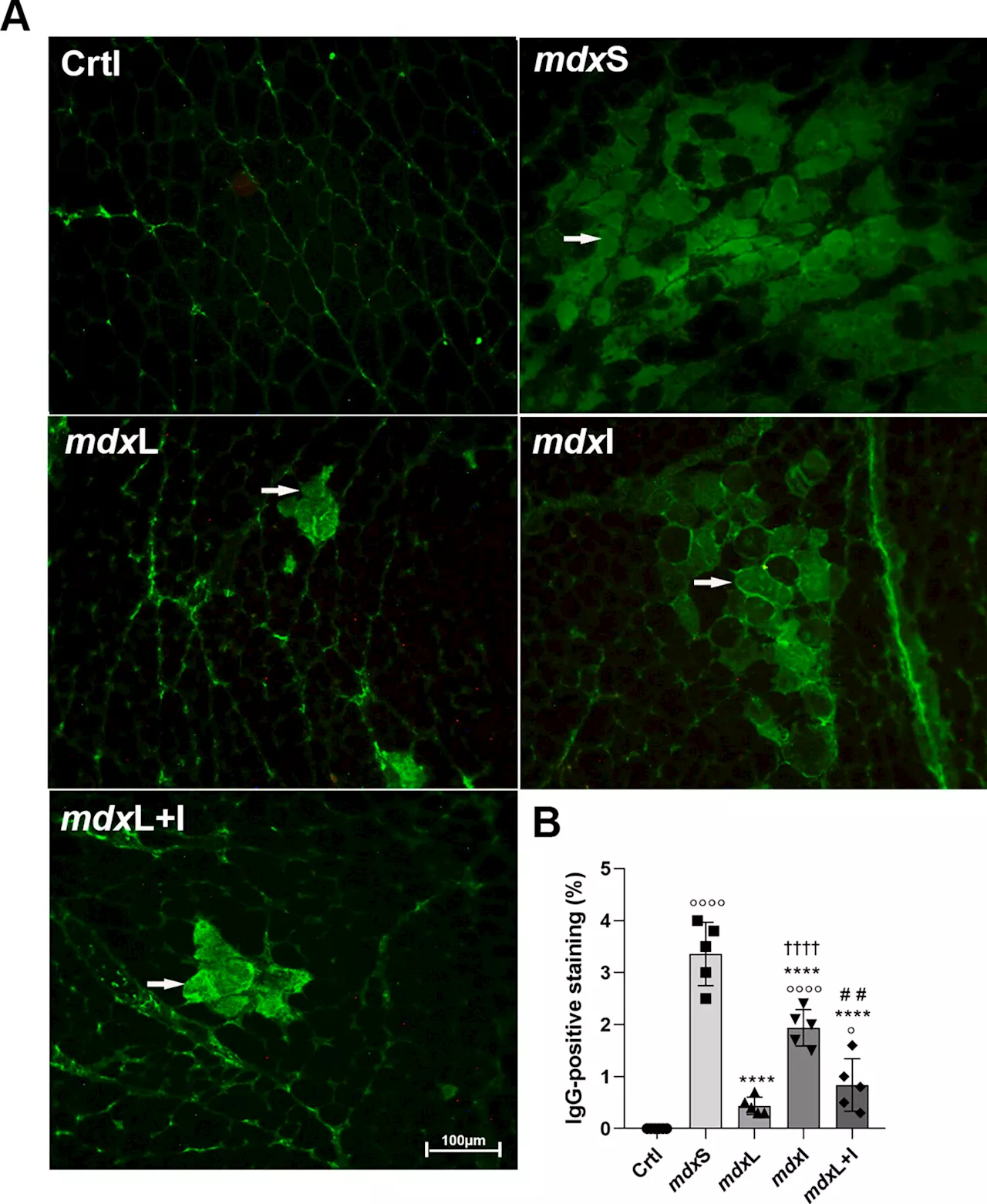 Study finds LED therapy and antioxidant drug benefit muscle regeneration in Duchenne muscular dystrophyIn an animal model of Duchenne muscular dystrophy, Brazilian researchers tested a therapy that combines photobiomodulation using laser light or light-emitting diodes (LEDs) with idebenone, an antioxidant compound investigated for application in neurodegenerative diseases.
Study finds LED therapy and antioxidant drug benefit muscle regeneration in Duchenne muscular dystrophyIn an animal model of Duchenne muscular dystrophy, Brazilian researchers tested a therapy that combines photobiomodulation using laser light or light-emitting diodes (LEDs) with idebenone, an antioxidant compound investigated for application in neurodegenerative diseases.
Read more »
 New therapeutic approach for Duchenne muscular dystrophy combines LED therapy with idebenoneIn an animal model of Duchenne muscular dystrophy, Brazilian researchers tested a therapy that combines photobiomodulation using laser light or light-emitting diodes (LEDs) with idebenone, an antioxidant compound investigated for application in neurodegenerative diseases.
New therapeutic approach for Duchenne muscular dystrophy combines LED therapy with idebenoneIn an animal model of Duchenne muscular dystrophy, Brazilian researchers tested a therapy that combines photobiomodulation using laser light or light-emitting diodes (LEDs) with idebenone, an antioxidant compound investigated for application in neurodegenerative diseases.
Read more »
 FDA official: The risk of secondary cancer from CAR-T therapy is less than fearedThe risk of secondary cancer after CAR-T therapy, pioneered at Penn, is less than regulators feared last year, an FDA official said June 14 at cell and gene therapy conference in King of Prussia.
FDA official: The risk of secondary cancer from CAR-T therapy is less than fearedThe risk of secondary cancer after CAR-T therapy, pioneered at Penn, is less than regulators feared last year, an FDA official said June 14 at cell and gene therapy conference in King of Prussia.
Read more »
 Continuous glucose monitors on the rise after FDA approvalContinuous glucose monitors are on the rise. But do we need them?
Continuous glucose monitors on the rise after FDA approvalContinuous glucose monitors are on the rise. But do we need them?
Read more »
 GSK’s £1.2bn Arexvy vaccine wins FDA approval for younger age rangeThe drug to treat RSV, a virus which causes 14,000 deaths annually in the US, is a key money-spinner for GSK.
GSK’s £1.2bn Arexvy vaccine wins FDA approval for younger age rangeThe drug to treat RSV, a virus which causes 14,000 deaths annually in the US, is a key money-spinner for GSK.
Read more »
 FDA gives nod to RSV vaccine for people in their 50sThe U.S. Food and Drug Administration on Friday has for the first time approved the use of a respiratory syncytial virus (RSV) vaccine for people in their 50s who are at increased risk for the illness.
FDA gives nod to RSV vaccine for people in their 50sThe U.S. Food and Drug Administration on Friday has for the first time approved the use of a respiratory syncytial virus (RSV) vaccine for people in their 50s who are at increased risk for the illness.
Read more »
