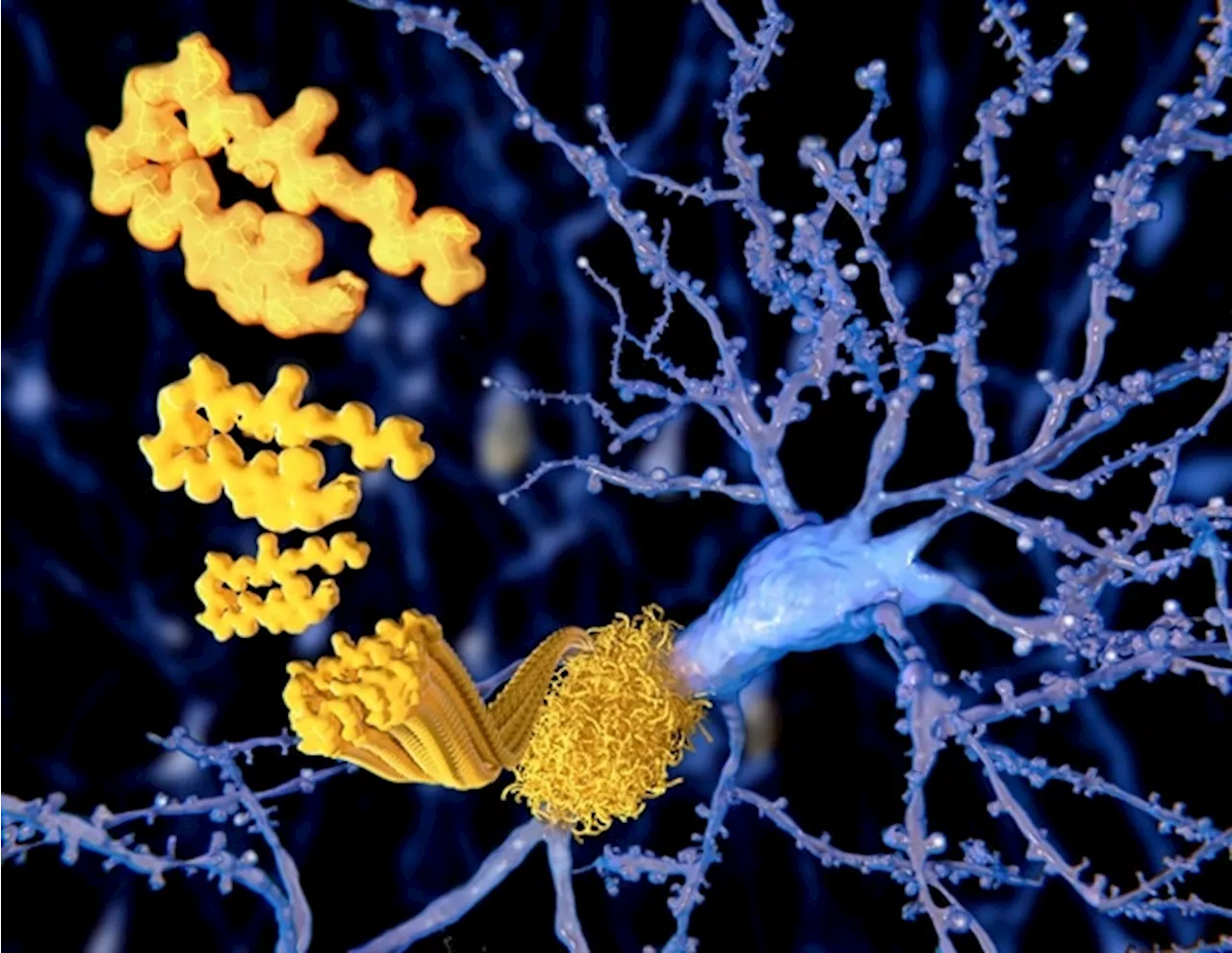
The Medicines and Healthcare products Regulatory Agency (MHRA) has today, 23 October 2024, approved a license for the medicine donanemab (Kisunla) for use in the early stages of Alzheimer’s disease, following a thorough review of the benefits and risks.
Medicines and Healthcare products Regulatory Agency Oct 26 2024 The Medicines and Healthcare products Regulatory Agency has today, 23 October 2024, approved a license for the medicine donanemab for use in the early stages of Alzheimer’s disease, following a thorough review of the benefits and risks.
We’re assured that, together with the conditions of the license approval, the appropriate regulatory standards for this medicine have been met. At week 76 of the study, patients treated with donanemab had statistically significantly less clinical progression in their Alzheimer’s disease compared to patients that were treated with the placebo. This was assessed by change in iADRS score from baseline. Patients with low to medium levels of tau protein showed 35% slowing of clinical progression which equated to 4.4 months of delay in disease progression.
Use of donanemab in patients who are on anticoagulants or have been diagnosed with cerebral amyloid angiopathy on MRI before starting treatment is contraindicated as the risks in these patients are considered to outweigh the benefits.
Apolipoprotein Brain Dementia Doctor Efficacy Gene Genes Healthcare Intracerebral Hemorrhage Medicine Placebo Protein
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 The 4 common medicines that ‘increase your chance of erectile dysfunction’What is Erectile Dysfunction?
The 4 common medicines that ‘increase your chance of erectile dysfunction’What is Erectile Dysfunction?
Read more »
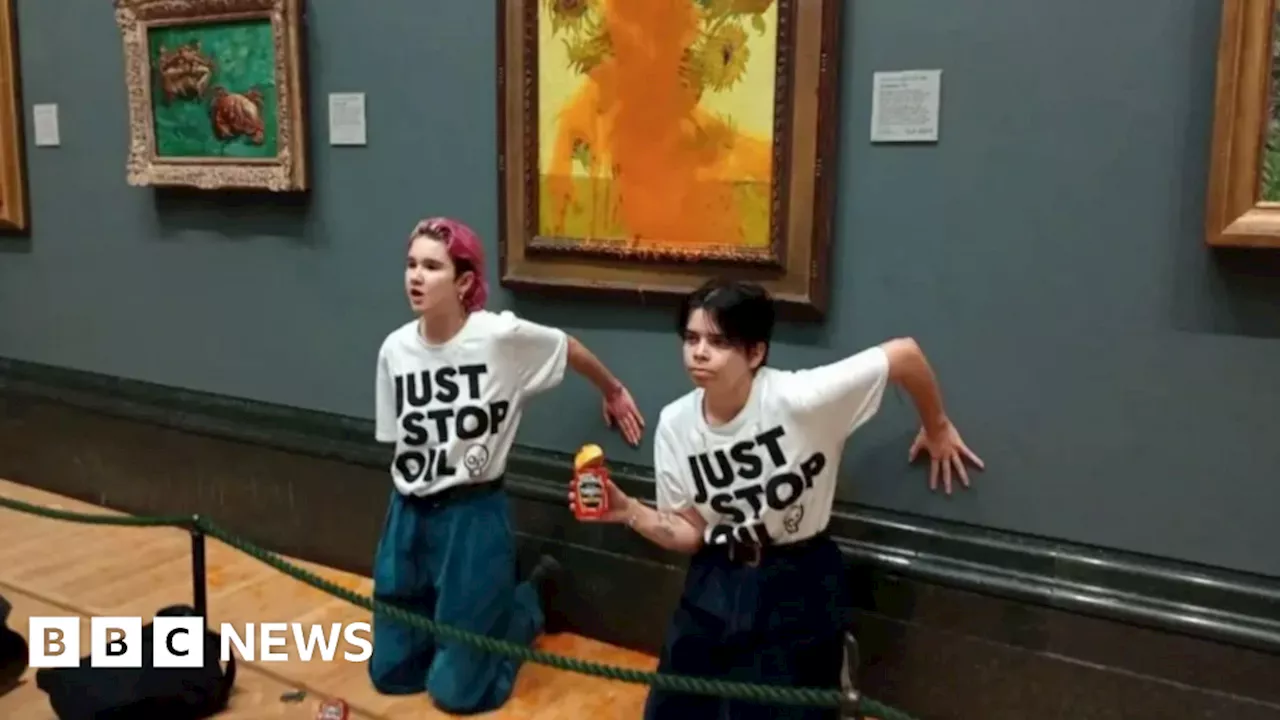 National Gallery bans liquids after repeated protestor attacksThe ban starts Friday morning and excludes baby formula, expressed milk and prescription medicines.
National Gallery bans liquids after repeated protestor attacksThe ban starts Friday morning and excludes baby formula, expressed milk and prescription medicines.
Read more »
 Exclusion of indigenous voices hinders HIV progress in Latin AmericaIndigenous communities in Latin America say they are being excluded from the global HIV/AIDS response, leaving them without access to life-saving medicines and prevention tools.
Exclusion of indigenous voices hinders HIV progress in Latin AmericaIndigenous communities in Latin America say they are being excluded from the global HIV/AIDS response, leaving them without access to life-saving medicines and prevention tools.
Read more »
 New Alzheimer’s drug donanemab rejected for widespread use in NHSThe news comes as the UK’s medicines regulator said donanemab could be licensed for use in the UK
New Alzheimer’s drug donanemab rejected for widespread use in NHSThe news comes as the UK’s medicines regulator said donanemab could be licensed for use in the UK
Read more »
 Doctor's 'report now' warning to those with condition that affects millionsDr. Alison Cave, a product regulator at the Medicines and Healthcare Products Regulatory Agency, has stressed that prioritising patient safety is their main concern.
Doctor's 'report now' warning to those with condition that affects millionsDr. Alison Cave, a product regulator at the Medicines and Healthcare Products Regulatory Agency, has stressed that prioritising patient safety is their main concern.
Read more »
 Understanding proteasome function in Trichomonas vaginalis for drug developmentResearchers from IOCB Prague are furthering the understanding of how medicines work and what it takes to develop their most effective variants.
Understanding proteasome function in Trichomonas vaginalis for drug developmentResearchers from IOCB Prague are furthering the understanding of how medicines work and what it takes to develop their most effective variants.
Read more »