Study provides insights on stability of SARS-CoV-2 in biological fluids of animals SARSCoV2 Coronavirus Disease COVID biologicalfluids cat sheep whitetaileddeer VirusesMDPI KState
Since the emergence of SARS-CoV-2 in 2019, the virus has continuously evolved genetically, jumped species barriers, and further expanded its range of hosts. In addition, recent studies have detected interspecies transmission, such as infection of domestic pets as well as viral circulation among wildlife. However, understanding SARS-CoV-2 stability within animal biological fluids and their function in viral transmission is limited since prior studies mainly assessed human biological fluids.
The team collected salivary, fecal, and urinary samples from sheep, cats, and WTDs. Cytopathic impacts were assessed four days after SARS-CoV-2 inoculation, followed by the estimation of viral titer. The stability exhibited by SARS-CoV-2 variants of concern as examined in WTD fecal suspensions using SARS-CoV-2 Alpha, Delta, and Omicron VOCs, which were cultivated in Vero-transmembrane serine protease 2 cells. Each viral stock also underwent sequencing.
SARS-CoV-2 stability in pooled cat saliva , pooled cat feces , pooled cat 10% fecal suspension , pooled sheep saliva , pooled white-tailed deer saliva , and pooled white-tailed deer feces . Each biological fluid was spiked with 5 × 104 TCID50 of SARS-CoV-2 and incubated under indoor , summer , spring/fall , and winter conditions. At each time point, the virus was recovered and titrated on Vero E6 cells.
United Kingdom Latest News, United Kingdom Headlines
Similar News:You can also read news stories similar to this one that we have collected from other news sources.
 Study suggests saliva samples may be better indicators of SARS-CoV-2 persistence than nasal swabsIn a recent study published in the PLOS ONE journal, researchers at Rutgers New Jersey Medical School explored the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human saliva.
Study suggests saliva samples may be better indicators of SARS-CoV-2 persistence than nasal swabsIn a recent study published in the PLOS ONE journal, researchers at Rutgers New Jersey Medical School explored the persistence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human saliva.
Read more »
 XBB/XBB.1.5 subvariant of SARS-CoV-2 shows increased vaccine sensitivityThe study, currently available on the medRxiv* preprint server, indicates that omicron subvariant XBB/XBB.1.5 is more sensitive to vaccine-induced immunity than other co-circulating SARS-CoV-2 variants.
XBB/XBB.1.5 subvariant of SARS-CoV-2 shows increased vaccine sensitivityThe study, currently available on the medRxiv* preprint server, indicates that omicron subvariant XBB/XBB.1.5 is more sensitive to vaccine-induced immunity than other co-circulating SARS-CoV-2 variants.
Read more »
 Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2 - Nature CommunicationsThe role of soluble angiotensin converting enzyme 2 (sACE2) in SARS-CoV-2 infection is not well understood. Here, authors show that membrane type 1 matrix metalloproteinase (MT1-MMP) releases sACE2 to promote SARS-CoV-2 cell entry in vitro and in vivo, and the upregulation of MT1-MMP may contribute to increased susceptibility to SARS-CoV-2 infection in ageing.
Control of SARS-CoV-2 infection by MT1-MMP-mediated shedding of ACE2 - Nature CommunicationsThe role of soluble angiotensin converting enzyme 2 (sACE2) in SARS-CoV-2 infection is not well understood. Here, authors show that membrane type 1 matrix metalloproteinase (MT1-MMP) releases sACE2 to promote SARS-CoV-2 cell entry in vitro and in vivo, and the upregulation of MT1-MMP may contribute to increased susceptibility to SARS-CoV-2 infection in ageing.
Read more »
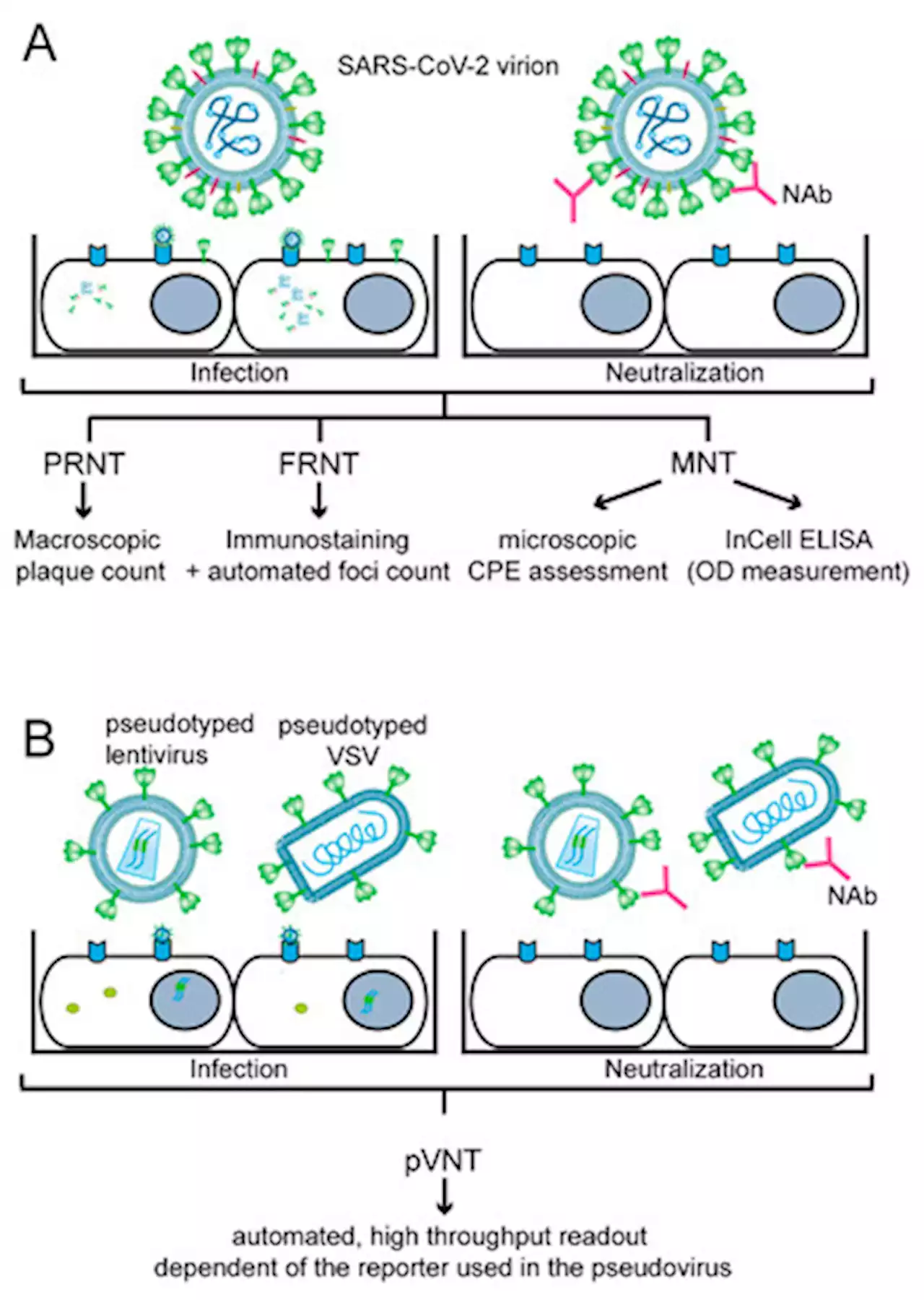 Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing AntibodiesMore than three years ago, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the unforeseen COVID-19 pandemic with millions of deaths. In the meantime, SARS-CoV-2 has become endemic and is now part of the repertoire of viruses causing seasonal severe respiratory infections. Due to several factors, among them the development of SARS-CoV-2 immunity through natural infection, vaccination and the current dominance of seemingly less pathogenic strains belonging to the omicron lineage, the COVID-19 situation has stabilized. However, several challenges remain and the possible new occurrence of highly pathogenic variants remains a threat. Here we review the development, features and importance of assays measuring SARS-CoV-2 neutralizing antibodies (NAbs). In particular we focus on in vitro infection assays and molecular interaction assays studying the binding of the receptor binding domain (RBD) with its cognate cellular receptor ACE2. These assays, but not the measurement of SARS-CoV-2-specific antibodies per se, can inform us of whether antibodies produced by convalescent or vaccinated subjects may protect against the infection and thus have the potential to predict the risk of becoming newly infected. This information is extremely important given the fact that a considerable number of subjects, in particular vulnerable persons, respond poorly to the vaccination with the production of neutralizing antibodies. Furthermore, these assays allow to determine and evaluate the virus-neutralizing capacity of antibodies induced by vaccines and administration of plasma-, immunoglobulin preparations, monoclonal antibodies, ACE2 variants or synthetic compounds to be used for therapy of COVID-19 and assist in the preclinical evaluation of vaccines. Both types of assays can be relatively quickly adapted to newly emerging virus variants to inform us about the magnitude of cross-neutralization, which may even allow us to estimate the risk of becoming infected by new
Importance, Applications and Features of Assays Measuring SARS-CoV-2 Neutralizing AntibodiesMore than three years ago, the Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) caused the unforeseen COVID-19 pandemic with millions of deaths. In the meantime, SARS-CoV-2 has become endemic and is now part of the repertoire of viruses causing seasonal severe respiratory infections. Due to several factors, among them the development of SARS-CoV-2 immunity through natural infection, vaccination and the current dominance of seemingly less pathogenic strains belonging to the omicron lineage, the COVID-19 situation has stabilized. However, several challenges remain and the possible new occurrence of highly pathogenic variants remains a threat. Here we review the development, features and importance of assays measuring SARS-CoV-2 neutralizing antibodies (NAbs). In particular we focus on in vitro infection assays and molecular interaction assays studying the binding of the receptor binding domain (RBD) with its cognate cellular receptor ACE2. These assays, but not the measurement of SARS-CoV-2-specific antibodies per se, can inform us of whether antibodies produced by convalescent or vaccinated subjects may protect against the infection and thus have the potential to predict the risk of becoming newly infected. This information is extremely important given the fact that a considerable number of subjects, in particular vulnerable persons, respond poorly to the vaccination with the production of neutralizing antibodies. Furthermore, these assays allow to determine and evaluate the virus-neutralizing capacity of antibodies induced by vaccines and administration of plasma-, immunoglobulin preparations, monoclonal antibodies, ACE2 variants or synthetic compounds to be used for therapy of COVID-19 and assist in the preclinical evaluation of vaccines. Both types of assays can be relatively quickly adapted to newly emerging virus variants to inform us about the magnitude of cross-neutralization, which may even allow us to estimate the risk of becoming infected by new
Read more »
 Elevated mucosal immune response expedites COVID-19 recoveryElevated mucosal immune response expedites COVID-19 recovery Coronavirus Disease COVID ImmuneResponse Mucosal ResearchSquare KingsCollegeLon nih_nhlbi UniofOxford imperialcollege
Elevated mucosal immune response expedites COVID-19 recoveryElevated mucosal immune response expedites COVID-19 recovery Coronavirus Disease COVID ImmuneResponse Mucosal ResearchSquare KingsCollegeLon nih_nhlbi UniofOxford imperialcollege
Read more »
 Childhood common colds help protect against COVID-19In a recent study published in the Proceedings of the National Academy of Sciences, researchers assessed the association between cross-reactive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) CD4+ T cells generation and age.
Childhood common colds help protect against COVID-19In a recent study published in the Proceedings of the National Academy of Sciences, researchers assessed the association between cross-reactive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) CD4+ T cells generation and age.
Read more »
